HRV Influencing Factors
Heart Rate Variability (HRV) reflects our body’s relaxation system and how it influences our heart. It’s linked to our mental state, emotions, stress, health, and social interactions. We aim to understand what affects this connection. There are two main ideas: it affects how we think and connect with others. Genes play a role, but various factors can change this. By organizing and understanding these factors, we can grasp how this connection impacts our lives and improve our understanding. We’re using a smart way to organize this information based on how our bodies and the world work together (see the references, 1.-65.).
Unifying Conceptual Framework of Factors Influencing Cardiac Vagal Control
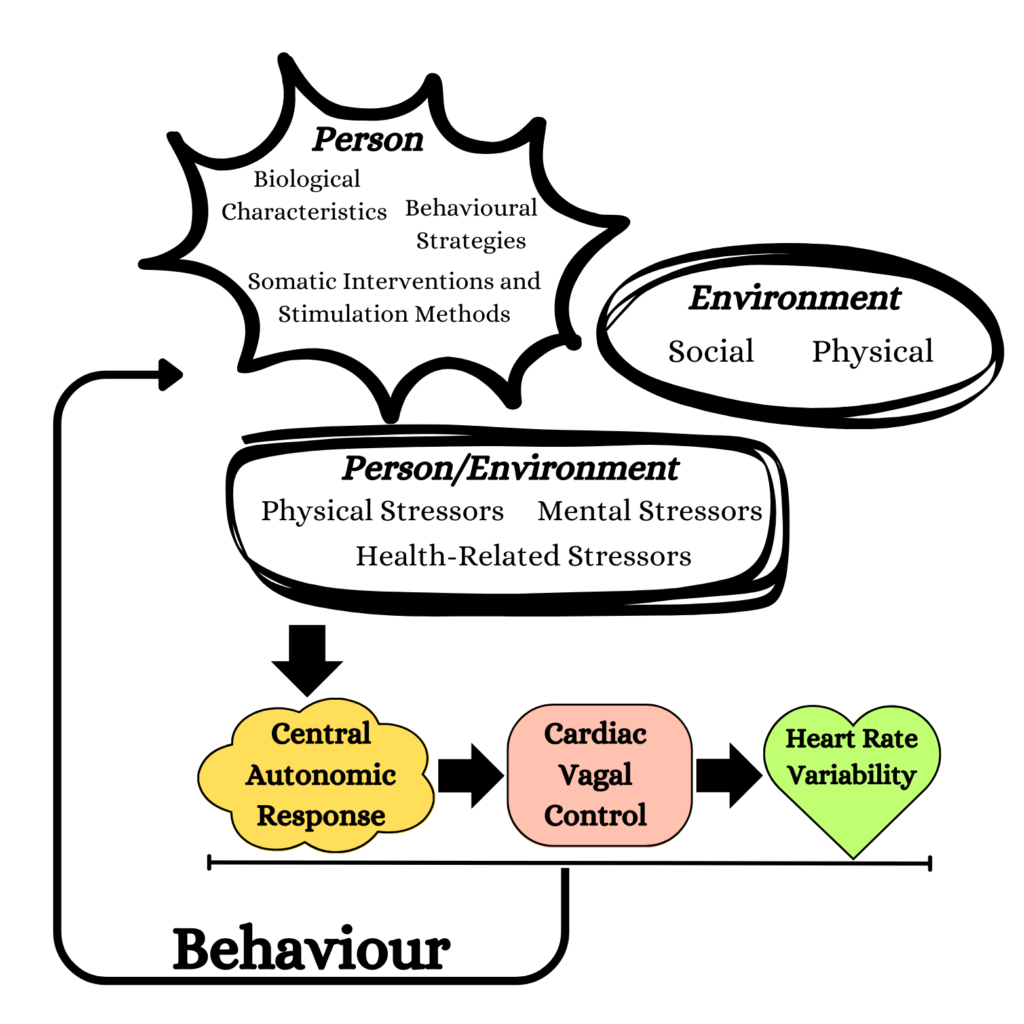
This figure shows how our body’s “coach” (the central autonomic network) talks to our heart. The coach considers lots of things like our feelings, surroundings, and our body’s abilities. It then guides our heart on how to beat effectively, known as cardiac vagal control (CVC). This control is crucial for how well we handle life’s challenges. The figure helps us see how all these parts work together and affect how we feel and act (1.).
Cardiac Vagal Control (CVC) (1.):
- Represents how the vagus nerve influences heart activity.
- Reflects the heart’s ability to respond and adapt.
Heart Rate Variability (HRV):
- Measures the variation in time intervals between heartbeats.
- Indicates the flexibility of the autonomic nervous system.
Connection:
- CVC controls the heart via the vagus nerve.
- Higher CVC leads to increased HRV, signifying a more adaptable autonomic system.
Person
Biological Factors (2.-13.):
- Stable Characteristics (2.-5.): Gender, age, body composition (amount of fat and muscle), ethnicity, and genetics influence HRV. Generally, women have higher HRV than men, and HRV tends to decrease with age. More body fat is linked to lower HRV.
- Transient Characteristics (6.-13.): Temporary factors like weight loss, body position (sitting, standing, lying down), sleep patterns, bladder fullness, breathing, blood pressure, body temperature, and hormones can affect HRV.
Somatic Interventions and Stimulation (14.-20.):
- Pharmacologic Factors (14.,15.): Certain medicines and substances can either increase or decrease HRV. Examples include medications for depression, high blood pressure, and substances like alcohol.
- Vagus Nerve Stimulation (16.,19.): Directly stimulating a nerve called the vagus nerve (which helps control many body functions) can increase HRV. This can be done through non-invasive methods too.
- Brain Stimulation (17.,18.): Techniques that involve stimulating the brain, such as using magnets or electrical currents, can affect HRV by changing how the brain works.
- Other Interventions (20.): Techniques like applying pressure to certain neck areas, stimulating the esophagus, breathing in oxygen, and using certain devices to aid breathing can influence HRV.
Behavioral Strategies (21.-37.):
- Nutrition (21.-28.): What you eat and drink can affect HRV. Some foods and supplements, like fatty fish and certain vitamins, can increase HRV.
- Non-Ingestive Oral Habits (29.-30.): Habits related to the mouth, like smoking or chewing gum, can lower HRV.
- Other Strategies (31.-37.): Activities like being in water, cooling the body, getting enough good-quality sleep, using relaxation techniques, practicing specific breathing patterns, engaging in music, and regular exercise can positively affect HRV.
Environment
Social Environment (38.-41.):
- Contact with Humans (38.-40.):
- When we’re close to people, especially during early stages of life, our HRV tends to be higher. Physical touch and support from loved ones can boost HRV.
- Contact with Animals (41.):
- Spending time with animals, like having a pet or going for a walk with a dog, can also increase our HRV. Animals can have a very positive effect on how our body responds.
- Overall, being around people and animals is good for our HRV, making us feel better and healthier.


Physical Environment (42.-53.):
- Aromas (42.,43.):
- Certain scents, like lavender or pine tree aromas, can make our HRV go up. They have a calming effect on our body.
- Lights (44.):
- Being exposed to natural or bright light can enhance HRV, especially if we’re feeling down or depressed.
- Sounds (excluding music) (45.,46.):
- Pleasant sounds like nature can improve HRV, while unpleasant sounds can make it go down. It’s important to pay attention to the sounds around us.
- Temperature (47.,48.):
- Being in a cool or moderate temperature environment is better for our HRV. Very hot environments can bring it down.
- Outdoor Environment (49.,50.):
- Being in nature, like a forest or park, can boost HRV. Fresh air and green spaces are good for our well-being.
- Altitude (51.-53.):
- Living in higher places affects HRV. Initially, it might decrease, but over time, it can return to normal or even become better for our health.
Person/Environment
Physical stressors (54.):
- Involve physical demands on the body that trigger a vagal withdrawal, preparing the body for the fight or flight response.
- Physical stressors include exercise, fatigue, and high or low ambient temperatures.
Mental stressors (55.-57.):
- Arise from the person’s perception of demands exceeding their resources, affecting CVC and being associated with emotional and cognitive challenges.
- Mental stressors include anxiety, trauma, and pressure from various sources.
Health-related stressors (58.-62.):
- Include pain, inflammation, and fatigue, as well as symptoms, syndromes, disorders, and diseases.
- Diseases like cardiovascular diseases, diabetes, and cancer are linked to altered CVC and can serve as indicators for medical risks.
Addictions (63.-65.):
- Addictions, characterized by continued use despite adverse consequences, are linked to self-regulation dysfunction and lower CVC.
- Examples include substance abuse, nicotine dependence, and internet addiction.
Summary

References
- Laborde, S., Mosley, E., & Mertgen, A. (2018). A unifying conceptual framework of factors associated to cardiac vagal control
- Antelmi, I., de Paula, R.S., Shinzato, A.R., Peres, C.A., Mansur, A.J., Grupi, C.J., (2004). Influence of age, gender, body mass index, and functional capacity on heart rate vari- ability in a cohort of subjects without heart disease
- Kim, J.A., Park, Y.G., Cho, K.H., Hong, M.H., Han, H.C., Choi, Y.S., Yoon, D., (2005). Heart rate variability and obesity indices: emphasis on the response to noise and standing
- Hill, L.K., Hu, D.D., Koenig, J., Sollers 3rd, J.J., Kapuku, G., Wang, X., Thayer, J.F., (2015). Ethnic differences in resting heart rate variability: a systematic review and meta-analysis
- Golosheykin, S., Grant, J.D., Novak, O.V., Heath, A.C., Anokhin, A.P., (2017). Ge- netic influences on heart rate variability
- Sjoberg, N., Brinkworth, G.D., Wycherley, T.P., Noakes, M., Saint, D.A., (2011). Moderate weight loss improves heart rate variability in overweight and obese adults with type 2 diabetes
- Young, F.L., & Leicht, A.S., (2011). Short-term stability of resting heart rate variability: influence of position and gender
- Boudreau, P., Yeh, W.H., Dumont, G.A., Boivin, D.B., (2012). A circadian rhythm in heart rate variability contributes to the increased cardiac sympathovagal response to awakening in the morning
- Heathers, J.A., Quintana, D., Angus, D., Krygier, J.R., Kemp, A.H., de Rosnay, M., (2018). Water Consumption as a Source of Error in the Measurement of Heart Rate Variability
- Purves, D., Augustine, G.J., Fitzpatrick, D., Katz, L.C., LaMantia, A.-S., McNamara, J.O., Williams, S.M., (2004). Neuroscience
- Eckberg, D.L., Eckberg, M.J., (1982). Human sinus node responses to repetitive, ramped carotid baroreceptor stimuli
- Carrillo, A.E., Cheung, S.S., Flouris, A.D., (2013). Autonomic nervous system modulation during accidental syncope induced by heat and orthostatic stress
- Armour, J.A., (2008). Potential clinical relevance of the ’little brain’ on the mammalian heart
- Elghozi, J.L., Girard, A., Laude, D., (2001). Effects of drugs on the autonomic control of short-term heart rate variability
- Desai, M.Y., Watanabe, M.A., Laddu, A.A., Hauptman, P.J., (2011). Pharmacologic modulation of parasympathetic activity in heart failure
- Vonck, K., Raedt, R., Naulaerts, J., De Vogelaere, F., Thiery, E., Van Roost, D., Boon, P., (2014). Vagus nerve stimulation…25 years later! what do we know about the effects on cognition?
- Gulli, G., Tarperi, C., Cevese, A., Acler, M., Bongiovanni, G., Manganotti, P., (2013). Effects of prefrontal repetitive transcranial magnetic stimulation on the auto- nomic regulation of cardiovascular function
- Rudorfer, M.V., Henry, M.E., Sackeim, H.A., (2003). Psychiatry. In: Tasman, A., Kay, J., Lieberman, J.A. (Eds.), Electroconvulsive Therapy
- Fallen, E.L., Kamath, M.V., Tougas, G., Upton, A., (2001). Afferent vagal modulation. Clinical studies of visceral sensory input
- Zannin, E., Pellegrino, R., Di Toro, A., Antonelli, A., Dellaca, R.L., Bernardi, L., (2015). Parasympathetic Stimuli on Bronchial and Cardiovascular Systems in Humans
- Park, S.K., Tucker, K.L., O’Neill, M.S., Sparrow, D., Vokonas, P.S., Hu, H., Schwartz, J., (2009). Fruit, vegetable, and fish consumption and heart rate variability: the Veterans Administration Normative Aging Study
- Hansen, A.L., Olson, G., Dahl, L., Thornton, D., Grung, B., Graff, I.E., Thayer, J.F., (2014). Reduced anxiety in forensic inpatients after a long-term intervention with Atlantic salmon
- Nakamura, H., Takishima, T., Kometani, T., Yokogoshi, H., (2009). Psychological stress-reducing effect of chocolate enriched with gamma-aminobutyric acid (GABA) in humans: assessment of stress using heart rate variability and salivary chromogranin
- Fu, C.H., Yang, C.C., Lin, C.L., Kuo, T.B., (2006). Effects of long-term vegetarian diets on cardiovascular autonomic functions in healthy postmenopausal women
- Richardson, T., Rozkovec, A., Thomas, P., Ryder, J., Meckes, C., Kerr, D., (2004). Influence of caffeine on heart rate variability in patients with long-standing type 1 diabetes
- Sagawa, Y., Kondo, H., Matsubuchi, N., Takemura, T., Kanayama, H., Kaneko, Y., Shimizu, T., (2011). Alcohol has a dose-related effect on parasympathetic nerve ac- tivity during sleep
- Quintana, D.S., Guastella, A.J., McGregor, I.S., Hickie, I.B., Kemp, A.H., (2013). Moderate alcohol intake is related to increased heart rate variability in young adults: implications for health and well-being
- Sucharita, S., Sowmya, S., Thomas, T., Kurpad, A.V., Vaz, M., (2013). Plasma vitamin B12, methylmalonic acid and heart rate variability in healthy young Indian adults
- Sjoberg, N., & Saint, D.A., (2011). A single 4 mg dose of nicotine decreases heart rate variability in healthy nonsmokers: implications for smoking cessation programs
- Shiba, Y., Nitta, E., Hirono, C., Sugita, M., Iwasa, Y., (2002). Evaluation of mastication-induced change in sympatho-vagal balance through spectral analysis of heart rate variability
- de Oliveira Ottone, V., de Castro Magalhaes, F., de Paula, F., Avelar, N.C., Aguiar, P.F., da Matta Sampaio, P.F., Rocha-Vieira, E., (2014). The effect of different water immersion temperatures on post-exercise parasympathetic reactivation
- Werner, G.G., Ford, B.Q., Mauss, I.B., Schabus, M., Blechert, J., Wilhelm, F.H., (2015). High cardiac vagal control is related to better subjective and objective sleep quality
- Garland, E.L., Froeliger, B., Howard, M.O., (2014). Effects of mindfulness-oriented recovery enhancement on reward responsiveness and opioid cue-reactivity
- Doufesh, H., Ibrahim, F., Ismail, N.A., Wan Ahmad, W.A., (2014). Effect of Muslim prayer (Salat) on alpha electroencephalography and its relationship with autonomic nervous system activity
- Iwanaga, M., Kobayashi, A., Kawasaki, C., (2005). Heart rate variability with repetitive exposure to music
- Rossi, F.E., Ricci-Vitor, A.L., Sabino, J.P., Vanderlei, L.C., Freitas Jr., I.F., (2014). Autonomic modulation and its relation with body composition in swimmers
- Stanley, J., Peake, J.M., Buchheit, M., (2013). Cardiac parasympathetic reactivation following exercise: implications for training prescription
- Bosquet Enlow, M., King, L., Schreier, H.M., Howard, J.M., Rosenfield, D., Ritz, T., Wright, R.J., (2014). Maternal sensitivity and infant autonomic and endocrine stress responses
- Maunder, R.G., Nolan, R.P., Hunter, J.J., Lancee, W.J., Steinhart, A.H., Greenberg, G.R., (2012). Relationship between social support and autonomic function during a stress protocol in ulcerative colitis patients in remission
- Gouin, J.P., Zhou, B., Fitzpatrick, S., (2015). Social integration prospectively predicts changes in heart rate variability among individuals undergoing migration stress
- Motooka, M., Koike, H., Yokoyama, T., Kennedy, N.L., (2006). Effect of dog- walking on autonomic nervous activity in senior citizens
- Matsumoto, T., Asakura, H., Hayashi, T., (2013). Does lavender aromatherapy alle- viate premenstrual emotional symptoms?: a randomized crossover trial
- Inoue, N., Kuroda, K., Sugimoto, A., Kakuda, T., Fushiki, T., (2003). Autonomic nervous responses according to preference for the odor of jasmine tea
- Grote, V., Kelz, C., Goswami, N., Stossier, H., Tafeit, E., Moser, M., (2013). Cardioautonomic control and wellbeing due to oscillating color light exposure
- Tkaczyszyn, M., Olbrycht, T., Makowska, A., Sobon, K., Paleczny, B., Rydlewska, A., Jankowska, E.A., (2013). The influence of the sounds of crying baby and the sounds of violence on haemodynamic parameters and autonomic sta- tus in young, healthy adults
- Krabs, R.U., Enk, R., Teich, N., Koelsch, S., (2015). Autonomic effects of music in health and Crohn’s disease: the impact of isochronicity, emotional valence, and tempo
- Peng, R.C., Yan, W.R., Zhou, X.L., Zhang, N.L., Lin, W.H., Zhang, Y.T., (2015). Time-frequency analysis of heart rate variability during the cold pressor test using a time-varying autoregressive model
- Sollers 3rd, J.J., Sanford, T.A., Nabors-Oberg, R., Anderson, C.A., Thayer, J.F., (2002). Examining changes in HRV in response to varying ambient temperature
- Horiuchi, M., Endo, J., Takayama, N., Murase, K., Nishiyama, N., Saito, H., Fujiwara, A., (2014). Impact of viewing vs. not viewing a real forest on physiological and psychological responses in the same setting
- Song, C., Ikei, H., Igarashi, M., Miwa, M., Takagaki, M., Miyazaki, Y., (2014). Physiological and psychological responses of young males during spring-time walks in urban parks
- Zhuang, J., Zhu, H., Zhou, Z., (2002). Reserved higher vagal tone under acute hypoxia in Tibetan adolescents with long-term migration to sea level
- Trimmel, K., (2011). Sensitivity of HRV parameters including pNNxx proven by short-term exposure to 2700 m altitude
- Lin, P.C., Chen, W.L., Kao, W.F., Yang, Y.H., Kuo, C.D., (2011). Effects of altitude in high-rise building on the autonomic nervous modulation in healthy subjects
- Stanley, J., Peake, J.M., Buchheit, M., (2013). Cardiac parasympathetic reactivation following exercise: implications for training prescription
- Beauchaine, T.P., Gatzke-Kopp, L.M., Mead, H.K., (2007). Polyvagal theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence
- Hjortskov, N., Rissen, D., Blangsted, A.K., Fallentin, N., Lundberg, U., Søgaard, K., (2004). The effect of mental stress on heart rate variability and blood pressure during computer work
- Tucker, P., Pfefferbaum, B., Jeon-Slaughter, H., Khan, Q., Garton, T., (2012). Emotional stress and heart rate variability measures associated with cardiovascular risk in relocated Katrina survivors
- Appelhans, B.M., Luecken, L.J., (2008). Heart rate variability and pain: associations of two interrelated homeostatic processes
- Tonhajzerova, I., Mokra, D., Visnovcova, Z., (2013). Vagal function indexed by respiratory sinus arrhythmia and cholinergic anti-inflammatory pathway
- Crosswell, A.D., Lockwood, K.G., Ganz, P.A., Bower, J.E., (2014). Low heart rate variability and cancer-related fatigue in breast cancer survivors
- Javorka, M., Trunkvalterova, Z., Tonhajzerova, I., Javorkova, J., Javorka, K., Baumert, M., (2008). Short-term heart rate complexity is reduced in patients with type 1 diabetes mellitus
- Lotufo, P.A., Valiengo, L., Bensenor, I.M., Brunoni, A.R., (2012). A systematic re- view and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs
- Thayer, J.F., Hall, M., Sollers, J.J., Fischer, J.E., (2006). Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: evidence for impaired inhibitory control of the HPA axis in heavy drinkers
- Lin, P.C., Kuo, S.Y., Lee, P.H., Sheen, T.C., Chen, S.R., (2014). Effects of internet addiction on heart rate variability in school-aged children
65. Ashare, R.L., Sinha, R., Lampert, R., Weinberger, A.H., Anderson, G.M., Lavery, M.E., McKee, S.A., (2012). Blunted vagal reactivity predicts stress- precipitated tobacco smoking
